Is the Pressure of Gas Independent of Its Temperature
A sample of a gas at room temperature occupies a volume of 180 L at a pressure of 262 torr. The temperature is associated with the average kinetic energy whereas the pressure is associated with something like the average of the absolute value of the momentum of the particles.

Ideal Gas An Overview Sciencedirect Topics
If the velocity of sound in a gas is 200 m s 1 when the temperature is 1 2 7 C then the velocity of sound in the same gas when temperature is increased by 9 0 0 F is.

. Level- At a given volume and temperature the pressure of a gas. According to Charles - GayLussacs Law the volume of a fixed amount of gas maintained at constant pressure is directly proportional to its temperature. C varies linearly as its mass.
The pressure of a gas is independent of how much gas is present. During an adiabatic process the pressure of a gas is found to be proportional to the cube of its absolute temperature. Or simply VT constant When the gas is compressed it means that the volume decreases.
No that is not correct. FracmM is simply the number of moles n of the gas. This problem has been solved.
Gases fill their containers completely. The enthalpy and internal energy of an ideal gas were asserted to be functions of temperature only. If you divide the room into two equal parts by a wall the pressure and temperature in each half will be the same though the mass of the air in each room is half of that for the entire room.
Temperature and pressure are both potential energy. The temperature or moles of gas what is the new volume V2. The pressure exerted by a gas on the walls of its container.
The amount of gaseous substance or the number of moles of gas. Let a third property float to achieve arbitrary values is not independence by my definition. Solid liquid or gas if you increase temperature with a.
Average kinetic energy triples. As Charles - GayLussacs Law states we could predict that the temperature of the gas would also decrease. O Gases can be compressed to a significant fraction of their volume.
O Gases completely fill any container into which they are placed. Start studying Gas Pressure. If the pressure is.
They are not independent as one affects the other. Here we can prove it using the property relations. The ratio CpCv for the gas is a 2 b 32 c 43 d 53.
Both temperature and pressure are associated with translation of the molecules at least in a gas. The product of the pressure and volume of an ideal gas is A A constant B Directly proportional to its temperature. A varies inversely as its mass c varies linearly as its mass b varies inversely as its square of its mass d is independent of its mass At constant volume the temperature of gas is increased.
At a given volume and temperature the pressure of a gas. D is independent of its. A graph of temperature and pressure is a.
Heating a balloon causes it to. The gas laws are a group of laws that govern the behaviour of gases by providing relationships between the following. Previously we said that the enthalpy of an ideal gas is independent of pressure at constant temperature.
No such gas actually exists but most simple gases exhibit this behavior at ordinary _____ and pressures. The gas laws were developed towards. C Inversely proportional to its temperature.
The volume occupied by a gas. At constant temperature the pressure of a gas is inversely proportional to its volume. A gas that exhibits simple predictable relationships among the variables of temperature volume and pressure is called aIn _____ gas.
If certain amount of a gas occupies a volume of 30 cc and exerts a pressure of 85 gcm square then find the difference in its pressure when the same amount of. A varies inversely as its mass. What relationship exits between gas pressure and temperature.
The absolute temperature of the gas. Gas pressure and temperature have a direct relationship. The gases of a gas is independent of its temp.
Then number of collisions. And the internal energy of an ideal gas is independent of volume at constant temperature. Study sets textbooks questions.
The distance between the particles in a gas is very large. D is simply fracmV. O Gases freely mix with each other.
A graph that shows the pressure of a gas varies inversely with its volume under a constant temperature demonstrates Boyles law A graph that shows that the volume of a gas is directly proportional to its temperature under constant pressure demonstrates. The pressure of a gas increases when its temperature. Retagged Oct 9 2020 by Raghuveer01.
B varies inversely as the square of its mass. Equal to the universal gas constant. By what factor does the average kinetic energy of the molecules of a gas in an aerosol container increase when the temperature.
If the pressure changes to 1310 torr with no change in. Learn vocabulary terms and more with flashcards games and other study tools. So your expression DfracMPRT is simply PVnRT.
Ideal Gas Equation And Absolute Temperature Boyle S Law Derivation

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors
Relationship Between Pressure And Temperature Pediaa Com

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors
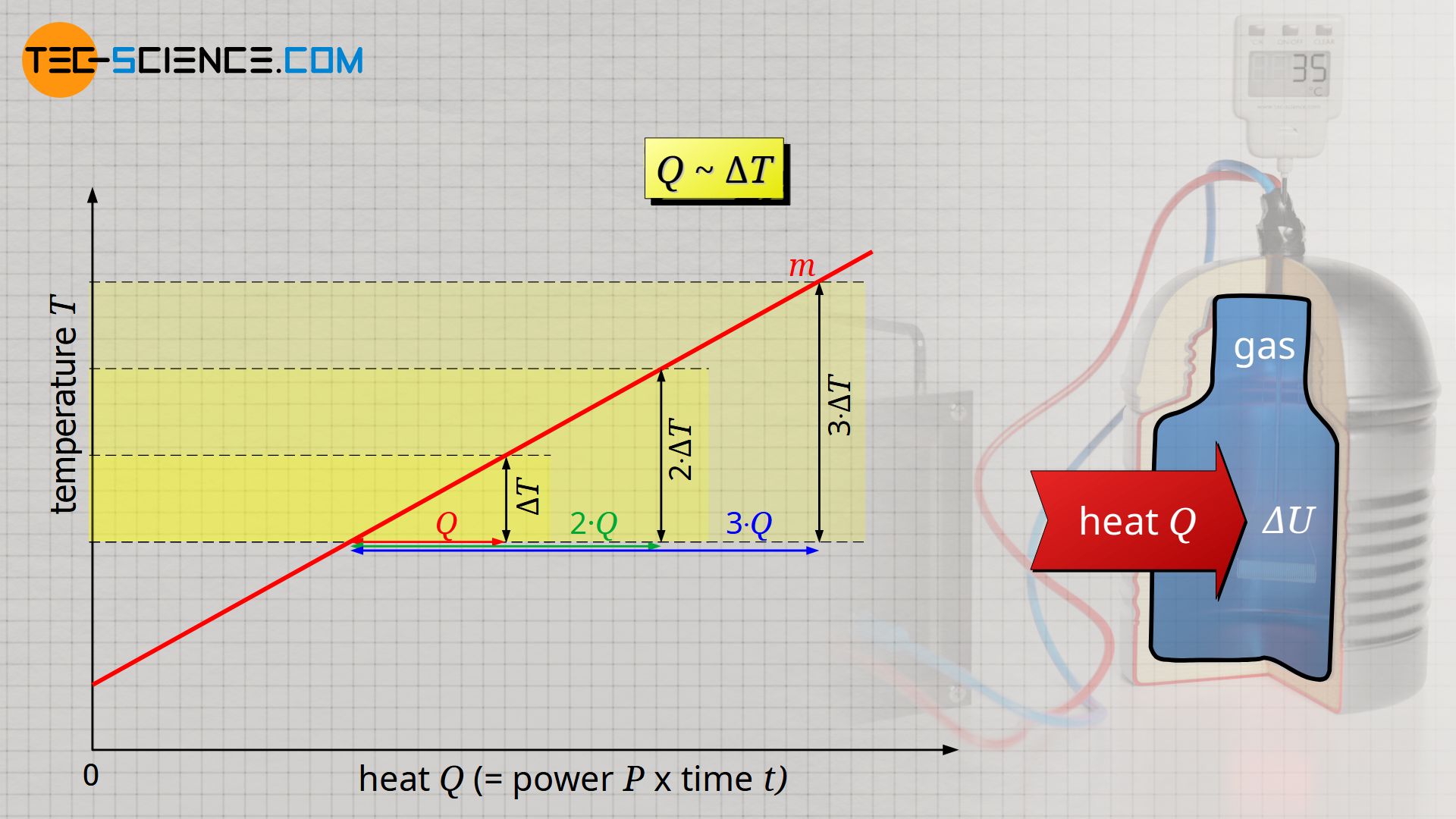
Calculation Of The Internal Energy For Ideal Gases Tec Science

Factors Affecting Gas Pressure Read Chemistry Ck 12 Foundation

Ideal Gas Equation And Absolute Temperature Boyle S Law Derivation
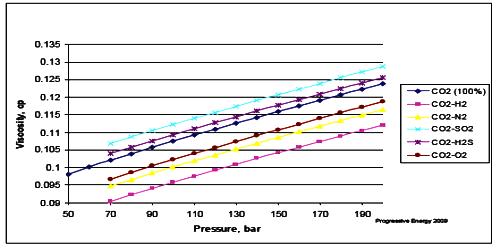
Effect Of Pressure On Viscosity Qs Study
The Gas Laws A Boyle S Law Boyle S Law States If The Temperature Of A Gas Sample Is Kept Constant The Volume Of The Sample Will Vary Inversely As The Pressure Varies This Statement Means That If The Pressure Increases The Volume Will Decrease If The
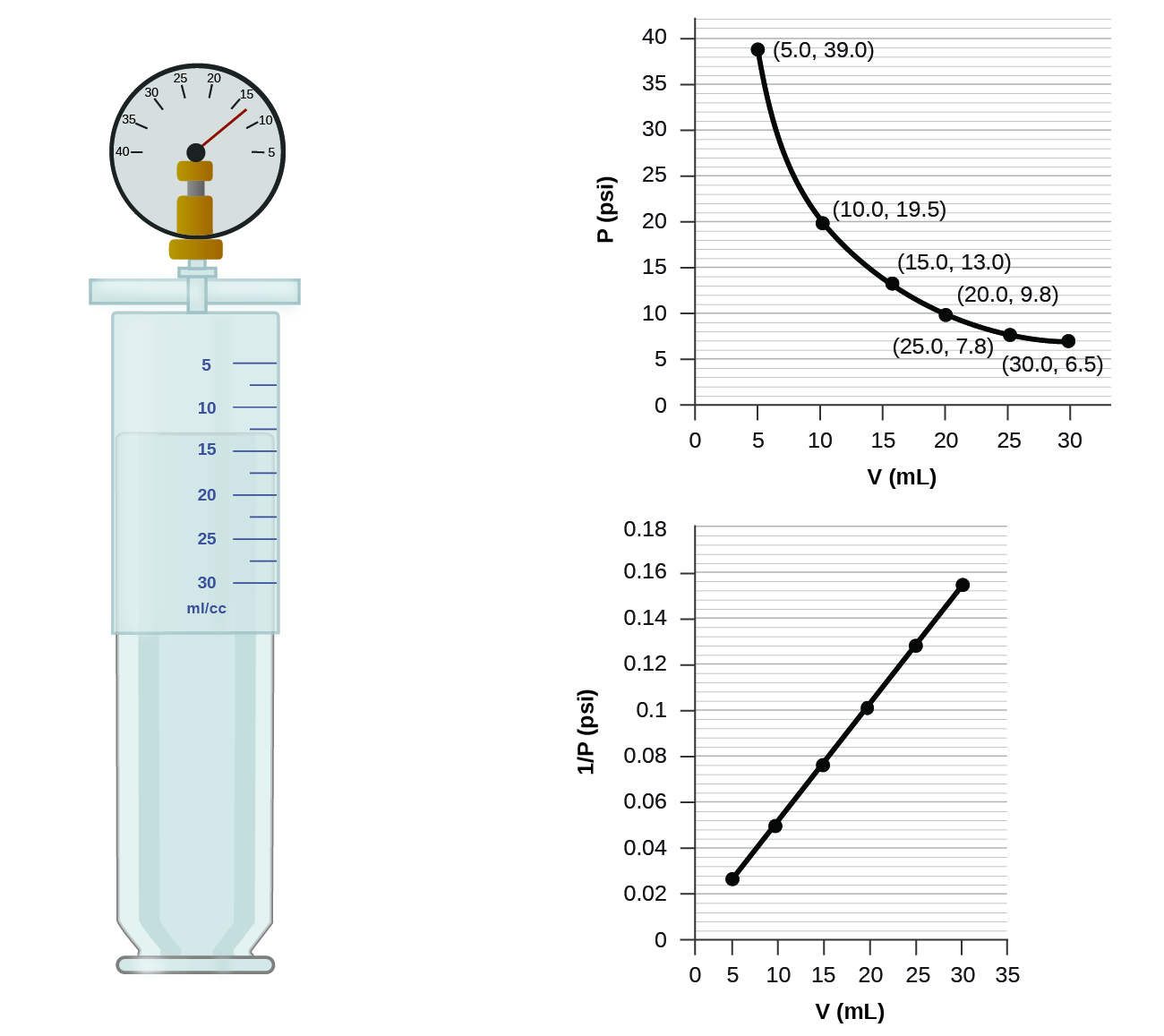
9 2 Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry
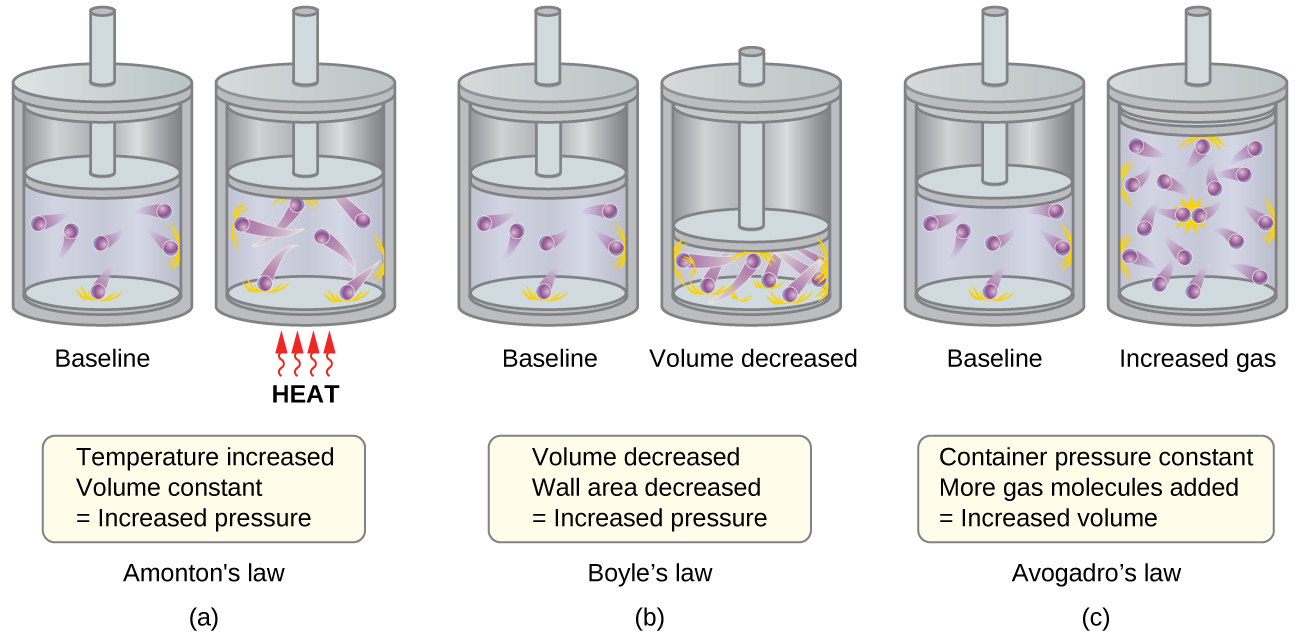
9 5 The Kinetic Molecular Theory Chemistry
The Gas Laws A Boyle S Law Boyle S Law States If The Temperature Of A Gas Sample Is Kept Constant The Volume Of The Sample Will Vary Inversely As The Pressure Varies This Statement Means That If The Pressure Increases The Volume Will Decrease If The
Ideal Gas Equation And Absolute Temperature Boyle S Law Derivation
Internal Energy And Enthalpy Of Ideal Gases Depend Only On Temperature
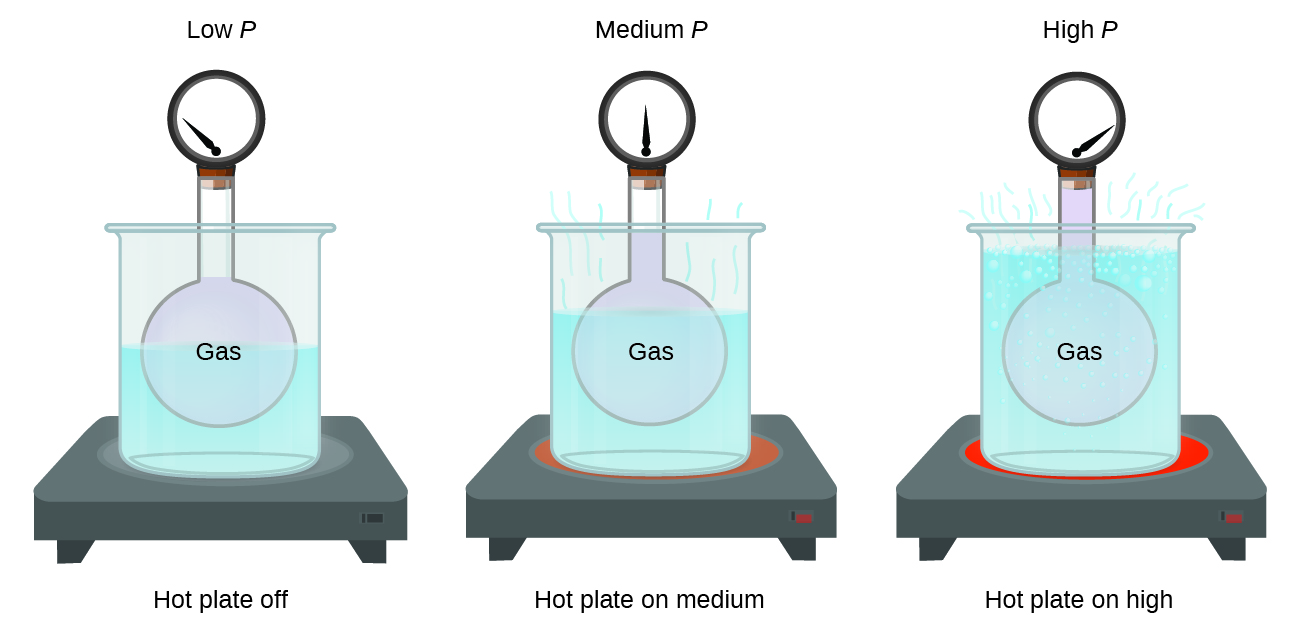
9 2 Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry


Comments
Post a Comment