Determine the Oxidizing Agent in the Following Reaction
Therefore V 2 0 5 is the oxidising agent. For example look at the following reaction.

Solved What Is The Oxidizing Agent In The Following Reaction Text Pyruvate Mathrm Nadh Mathrm H Rightarrow Text Lactate Mathrm Nad A Oxygen B Nadh C Lactate D Pyruvate
A chemical reaction between nickel and silver perchlorate.

. So the element with the highest electronegativity is the strongest oxidizing agent. 17 Determine the oxidizing agent in the following reaction. A Ag B Ni C Cl D O E This is not an oxidation-reduction reaction.
The oxidation state of Oxygen on the reactants side is-2 whereas on the products side is -3. Instead the oxidizing agent is assumed and can often be one of many different oxidizing agents. Nis 2 AgClO4aq NiClO42aq 2 Ags A Ag B Ni C CI D O E This is not an oxidation-reduction reaction.
2 Al 3 aq 2 Fe s 2 Al s 3 Fe 2 aq A Al3. 32 A beaker contains 050 mol of potassium bromide. 2M nO 4 5H 2SO3 M n2 5SO2 4 4H 3H 2O.
The oxidizing agent in the reaction is silver perchlorate. Chemistry questions and answers. Nis 2 AgC104aq NiC1042aq 2 Ags.
What is the oxidizing agent in the redox reaction represented by the following cell notation. Oxidation is the removal of electrons from an atom or polyatomic ion. This gain of electrons leads us to believe that H 2 O is the oxidising agent in the above reaction.
16 Identify the reducing agent. E This is not an oxidation-reduction reaction. Identify the oxidizing and reducing agents in the oxidation -reduction reaction.
Determine the oxidizing agent in the following reaction. Determine the oxidizing agent in the following reaction. 4 aq AlOH 4 aq MnO.
Correct answer - Determine the oxidizing agent in the following reaction. Ni s 2 AgClO4 aq Ni ClO42 aq 2 Ag s O AO OB Ni OCCI OD Ag E This is not an oxidation-reduction reaction. Ni s 2 AgClO 4 aq Ni ClO 4 2 aq 2 Ag s A Ag.
If yes name the oxidizing agent as well as reducing agent. Eq2I- rightarrow I_2 eq. From the following reactions determine if Ag is a stronger oxidizing agent than Cu 2.
CHOl2Cu 2aq5OH aq No Change observed. Write the reduction half reactions for all four reactions in. Ni s 2 AgClO4aq Ni ClO42aq 2 Ag s A Ag.
4 aq is the oxidizing agent. 18 When an aqueous solution of manganese II nitrate is combined with an aqueous solution of ammonium sulfide what should precipitate out. Chemistry questions and answers.
Als is the reducing agent. Nis 2 AgClO4aq NiClO42aq 2 Ags - ScieMce. Determine the oxidizing agent in the following reaction.
Sulfite SO2 3SI V is oxidized to sulfate SO2 4SV I and this sulfite serves as the reducing agent. Ni s 2 AgCIO4 aq Ni CIO42 aq 2 Ag s Determine the oxidizing agent in the following reaction. Determine what is oxidized and what is reduced.
E H 2 O is the oxidising agent. 4Al 3O₂ 2Al₂O₃. Ol Als MnO.
Which of the following is the oxidizing agent in the following reaction. Solution for determine the oxidizing agent in the following reaction Sn4 Ca -- Sn2 Ca2. 31 Determine the oxidizing agent in the following reaction.
Identify the oxidizing agent and the reducing agent in the following equation. Determine what is oxidized and what is reduced. 15 Determine the oxidizing agent in the following reaction.
Determine the oxidizing agent if any in the following reaction CaCO3 s 2 HNO3 aq CO2 g H2O l Ca NO32 aq HNO3 CO2 there is no oxidizing agent as this is not an oxidation-reduction reaction O H₂O CaCO3. E This is not an oxidation - reduction reaction. Determine the oxidizing agent in the following reaction 2 Li s Fe C2H3O22 aq 2 LiC2H3O2 aq Fe s Expert Solution.
Identifying Oxidizing and Reducing Agents. Mg 2HCl rightarrow MgCl_2 H_2 By signing up youll get thousands of. Mns Mn2aq Agaq Ags.
ZnsCu Zn Cus is this reaction redox reaction. The higher the electronegativity the greater the pull an oxidizing agent has for electrons. The higher the pull for electrons the stronger the oxidizing agent.
Nis 2 agclo4aq niclo42aq 2 ags. What conclusion about the compound N a 4 X e O 6 of which X e O 6 4 is a part can be drawn from the reaction. 4 Determine the oxidizing agent in the following reaction.
Determine the oxidizing agent reducing agent the substance being oxidized and the substance being reduced in each reaction in Part 1. Manganese as permanganate is reduced to the almost colourless M n2 so what you would see here is the reaction mix losing its deep purple colour at the endpoint. The oxidizing agent in the reaction.
Identify the oxidizing agent and the reducing agent in the following equation. The oxidation state of nickel in nickel metal 0The oxidation state of nickel in nickel perchlorate 2.
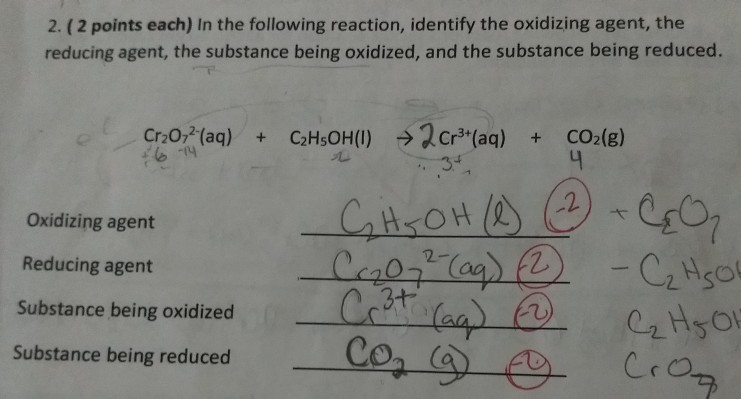
Solved 2 2 Points Each In The Following Reaction Chegg Com

Oxidizing Agents And Reducing Agents Youtube
Identify The Redox Reactions Out Of The Following Reactions And Identify The Oxidising And Reducing Agents In Them Sarthaks Econnect Largest Online Education Community

Solved Determine The Oxidizing Agent In The Following Chegg Com

Solved Identify The Oxidizing And Reducing Agents For The Chegg Com
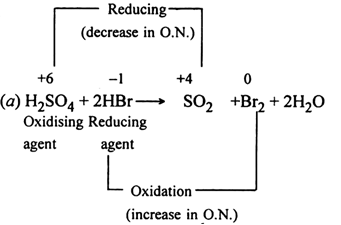
Identify The Oxidising Agent Reducing Agent And The Substance Undergoing Oxidation And Reduction In The Following Reactions From Chemistry Redox Reactions Class 11 Cbse

Lesson Explainer Oxidization And Reduction Nagwa

Select The Oxidising Agent And The Reducing Agent From The Following Reaction Underset Youtube

Identify The Substance Oxidised Reduced Oxidising Agent And Reducing Agent For The Following Reaction 2agbr S C6h6o2 Aq 2ag S 2h Br Aq C6h4o2 Aq
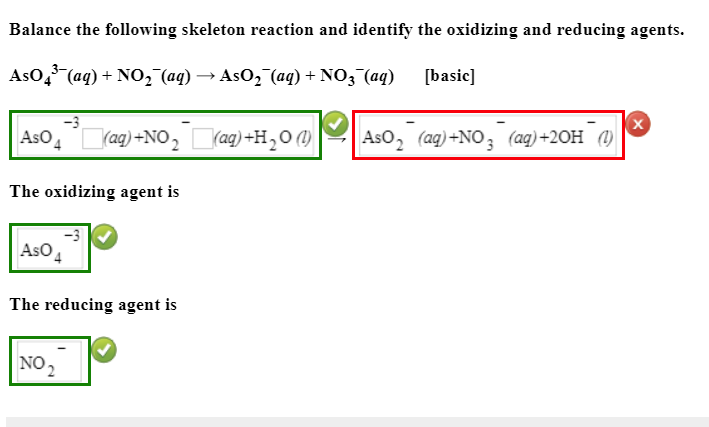
Solved Balance The Following Skeleton Reaction And Identify Chegg Com

Solved Determine The Oxidizing Agent In The Following Chegg Com
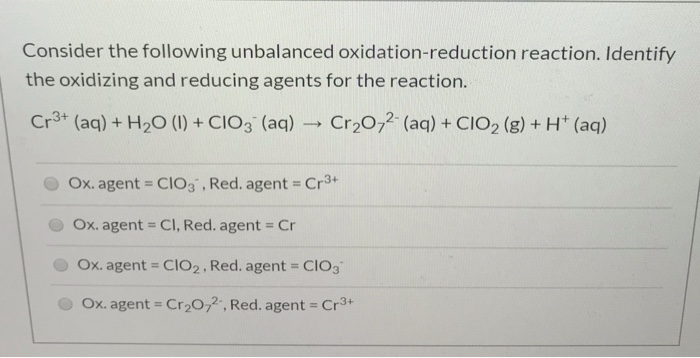
Solved Identify The Oxidizing And Reducing Agents For The Chegg Com

Name The Substance Oxidised And The Substance Reduced And Also Identify The Oxidising Agent And Reducing Agents In The Following Reaction A 3mno2 4al 3mn 2al2o3 B Fe2o3 3co Flash Education

Identify The Substance Oxidised Substance Reduced Oxidising Agent And Reducing Agent For Each Youtube

Name The Reducing Agent Oxidizing Agent And The Substance Oxidized And Reduced In The Following Reaction With Reason Nh3 O2 Gives No H2o Chemistry Topperlearning Com K018ecrr
Identify The Redox Reactions Out Of The Following Reactions And Identify The Oxidising And Reducing Agents In Them Sarthaks Econnect Largest Online Education Community
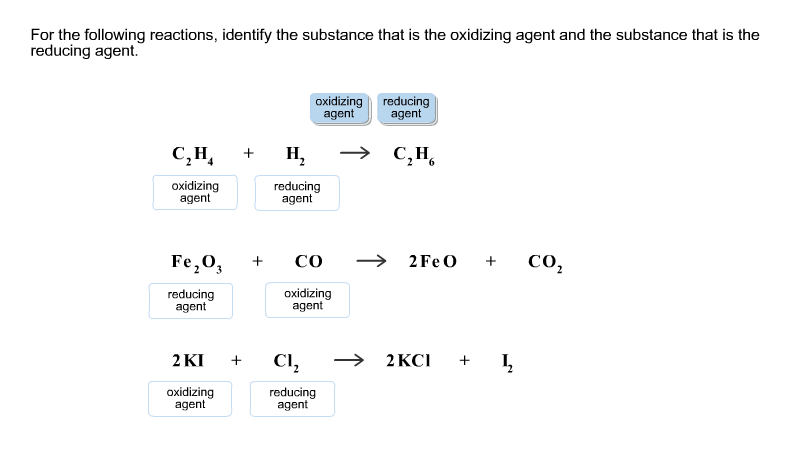
Solved For The Following Reactions Identify The Substance Chegg Com

Identify The Oxidizing Agent And The Reducing Agent In The Following Redox Reaction Mno 4 So 2 3 Mn 2 So 2 4

Identify The Oxidizing Agent And The Reducing Agent In The Following Redox Reaction Mno 4 So 2 3 Mn 2 So 2 4
Comments
Post a Comment